The recent FDA approval of Leqembi, the first new Alzheimer's treatment in two decades, marks a significant step forward in the fight against this debilitating disease. This innovative medication directly targets amyloid plaques in the brain, a key hallmark of Alzheimer's, and has demonstrated a 27% slowing of cognitive decline in clinical trials. Two Ohio residents who participated in these trials shared their personal experiences with Fox News Digital, offering a glimpse into the drug's potential impact.
Joan Murtaugh: 'Gradual Progress'
Joan Murtaugh, 77, of Lakewood, Ohio, began experiencing memory issues around her 70th birthday. Diagnosed with mild cognitive impairment in 2017, and later identified as having a high risk of developing Alzheimer's, she enrolled in the Leqembi clinical trial in 2020. While unsure whether she initially received the drug or a placebo, she believes she's benefited. "I am fully functional," Murtaugh shared. "I can still drive, shop, garden, cook, read – all those things." She continues to receive the medication, now via weekly injections, and has experienced no side effects. Her husband, Larry, describes Leqembi as "a ray of sunshine," and they remain optimistic about the future.
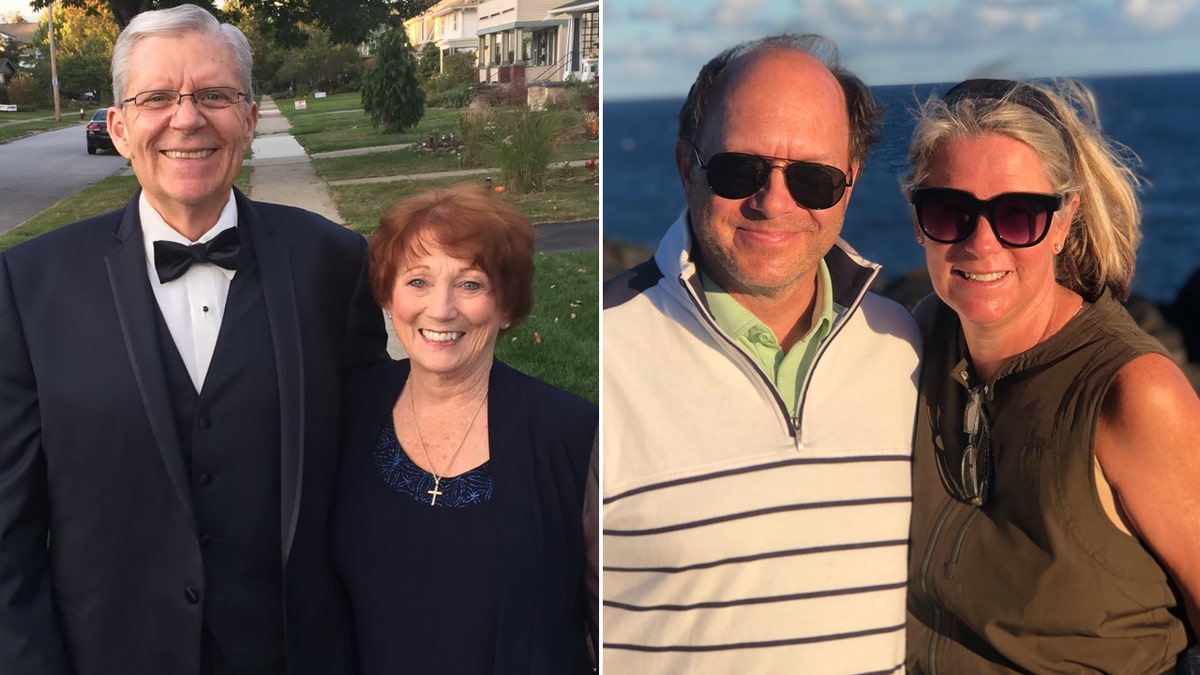
Joan Murtaugh (left) with her husband, Larry, and John Domeck (right) with his wife, Ann, participated in the Leqembi trials.

Joan and Larry Murtaugh's journey began with an appointment at a brain center clinic.

Joan Murtaugh has maintained her daily activities throughout the trial.
John Domeck: 'I Thank God Every Day'
John Domeck, a 61-year-old retired attorney from Aurora, Ohio, received his Alzheimer's diagnosis at the age of 57. His cognitive decline was noticed first by colleagues and family. After participating in the Leqembi trial, he's seen minimal progression of his symptoms. "The fact that he’s still able to maintain his day-to-day activities…is so promising," his wife, Ann, commented. John continues to drive, golf, and read. The Domecks credit the drug with giving them precious time together, allowing them to celebrate milestones like their son's wedding and plan trips to visit their daughter in Europe.

John Domeck with his family.
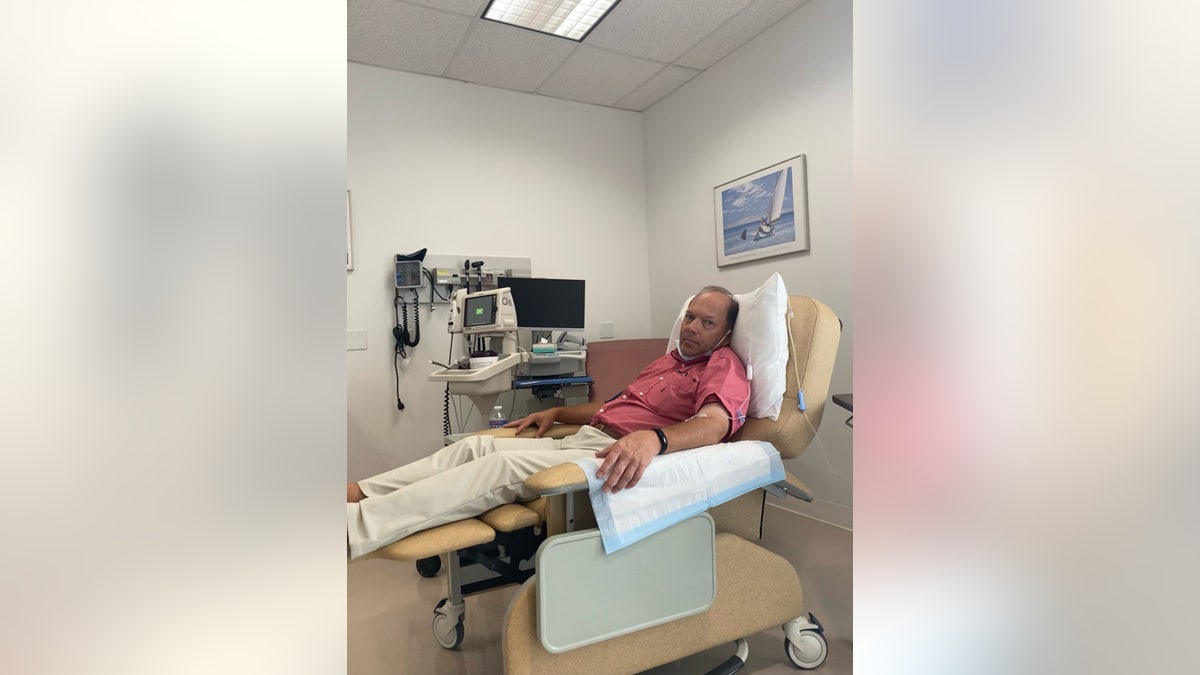
John Domeck receiving an infusion during the clinical trial.

John Domeck with his family at his daughter's graduation.

John Domeck continues to enjoy golfing.
About Leqembi
Leqembi targets the underlying cause of Alzheimer's, not just the symptoms. It works by prompting the immune system to clear amyloid plaques from the brain. While not a cure, it has shown promising results in slowing cognitive decline. The drug requires ongoing monitoring and is suitable for patients with mild cognitive impairment or early-stage Alzheimer's with confirmed amyloid plaques. Potential side effects include brain swelling and bleeding, and patients on blood thinners may not be eligible. With full FDA approval, Medicare will cover Leqembi, making it more accessible to patients. Experts emphasize that Leqembi is most effective when combined with other treatments and support systems.








